Distribution of Molecular Weights
Molecular weight is a criterion used to determine the size of the polymeric chain since greater molecular weight means greater size. Due to the statistical nature ruling the polymerization reactions, the size of the chains is the same for all of them.
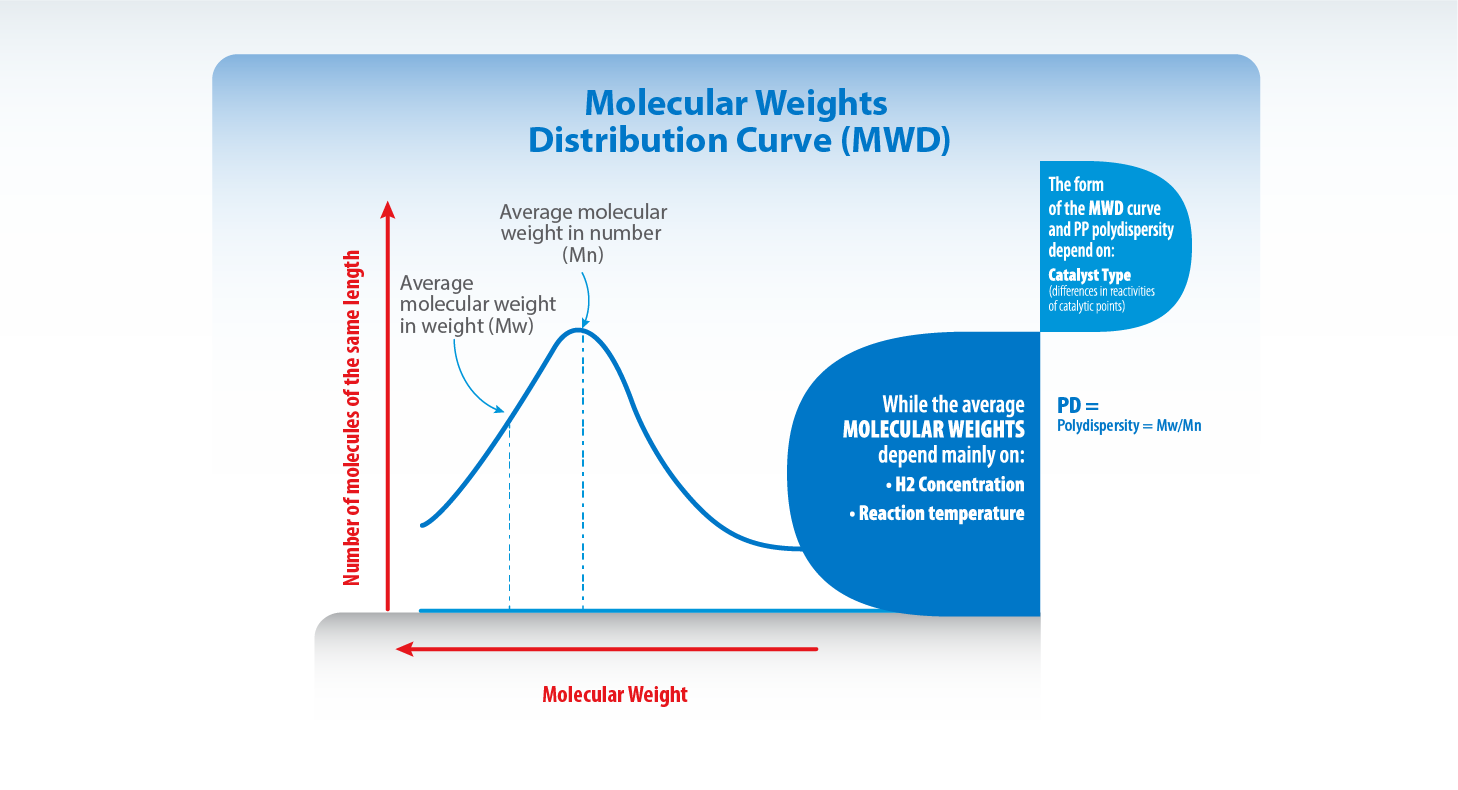
1 Molecular Weights Distribution Curve:
If the number of chains is graphed against its molecular weight, we will obtain the curve known as molecular weights distribution curve (MWD). The form of the MWD curve and the PP polydispersity depend on:
• Catalyst type (differences in reactivities of catalytic points).
The average molecular weights depend mainly on:
• H2 Concentration.
• Reaction temperature.
A- Average Molecular Weight in Number (Mn):
It is the total weight of all polymer molecules contained in a sample divided by the total number of polymer molecules of said sample (arithmetic average – all chains are equally important when calculating this parameter.)
B- Average Molecular Weight in Weight (Mw):
It is based on the fact that a bigger molecule contains more of the total weight of the polymer sample than smaller molecules (this parameter is highly susceptible to chains of high molecular weight).
C- Polydispersity (IP):
This measures the amplitude of the Molecular Weights Distribution Curve (MWD) and represents the ratio between the average molecular weight (Mw) and the average molecular weight in number (Mn).
2 Peroxide:
Peroxides are organic substances of low molecular weight that include in its chemical structure at least one peroxide group (oxygen-oxygen bond) which is decomposed by the action of heat generating free radicals (thermal degradation). Then, they act on the polymer chains causing their division or crosslinking. Depending on the polymer type being used, one of the mechanisms prevails over the other. In the specific case of polypropylene, the division of the chains is the most convenient one.
A- Controlled Rheology:
Controlled rheology implies polymers degradation (division of the chain) by using peroxides after the polymerization. In the particular case of the polypropylenes, the segments of greater molecular weight are the most affected ones during the process. Thus, it takes place a distribution of molecular weights (MWD) substantially narrower than the one present in the initial polymer (lower polydispersity index).
The resulting resin has lower elasticity degree. So, the performance of the equipments and the quality of finished units are improved in many aspects.
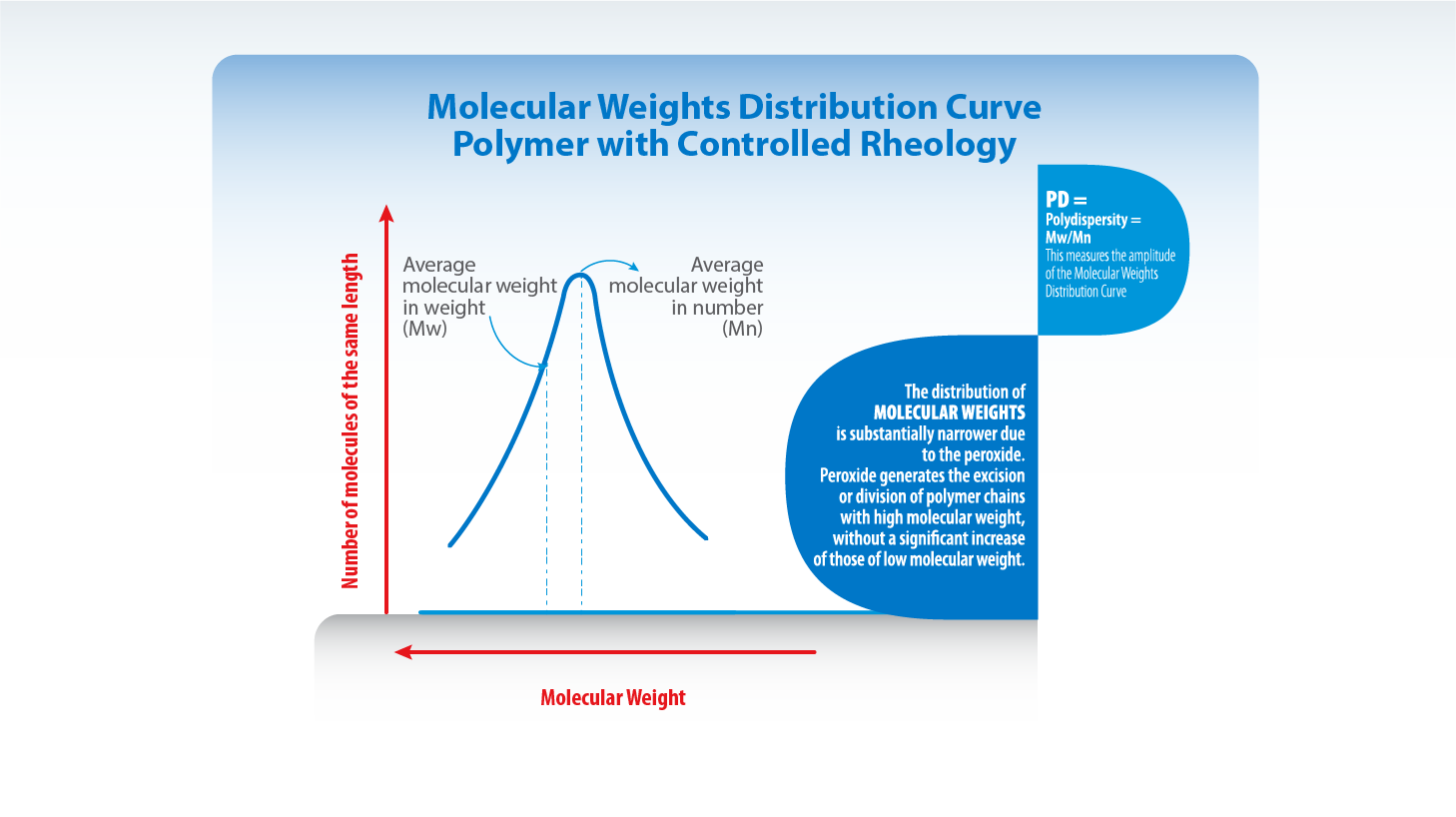
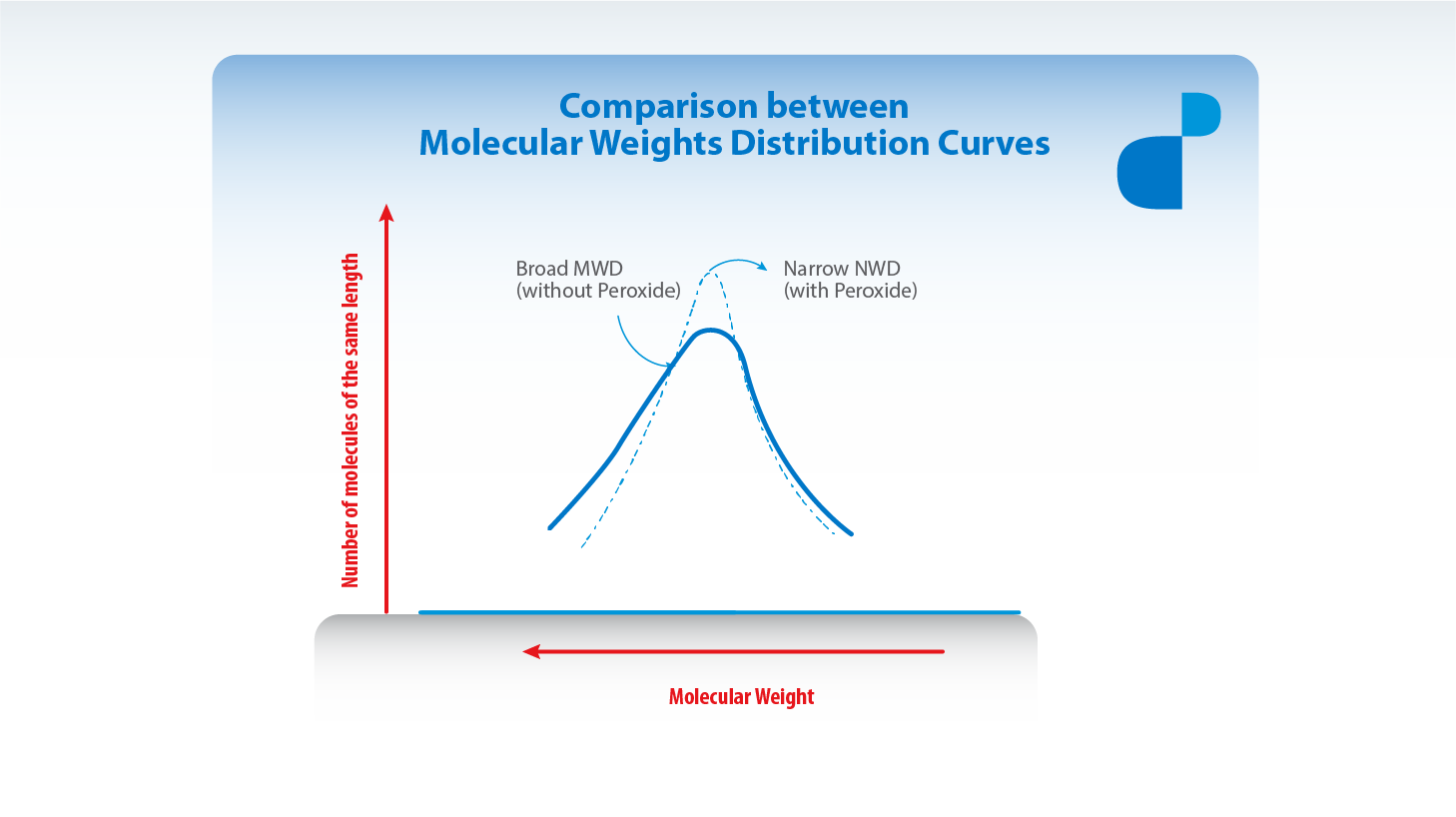
Many of the polymers’ properties are correlated with the average molecular weights and some others with the molecular weights distribution. CUYOLEN Controlled Rheology resins are relatively new materials in which the ends of high molecular weight have been removed without significant increase of the ends of low molecular weight. As a result, the distribution of molecular weights is substantially narrower (lower Polydispersity Index) and the resulting resin has lower elasticity degree. So, the performance of the equipments and the quality of finished units are improved in many aspects.
B- Relation between molecular weight and fluidity index.
As stated previously, the parameters defining a particular material are the average molecular weights and the distribution of molecular weights.
While the most common direct methods used to measure the average molecular weights are the Light Scattering (Mw) and Membrane Osmometry (Mn), the Distribution of Molecular Weights is calculated by Gels Permeation Chromatography.
As these direct methods have a high cost, an indirect measure of molecular weight is used in order to control the quality assurance of a certain product. Thus, for that purpose, we take advantage of that indirect relation existing between the molecular weight and the viscocity of the material, i. e. greater average molecular weight means greater viscocity.
More precisely, the Fluidity Index (IF, for its acronyms in Spanish) is what is determined. That Fluidity Index is the amount of material that flows through a capillary of established measures in certain conditions during a lapse of 10 minutes and that is the direct function of viscocity. For the same conditions of pressure drop in the capillary and melting temperature, a more viscose sample (of greater molecular weight) will show lower fluidity index. This index is known internationally as Melt Flow Index (MFI).
It is important to mention that the IF provides a measure of the average molecular weight of a certain product. It does not provide a measure regarding molecular weight distribution. There exist many behaviors detected in the polymers which are explained from the molecular weights distribution. Thus, two materials with identical IF may behave in a very different way during the transformation process.
